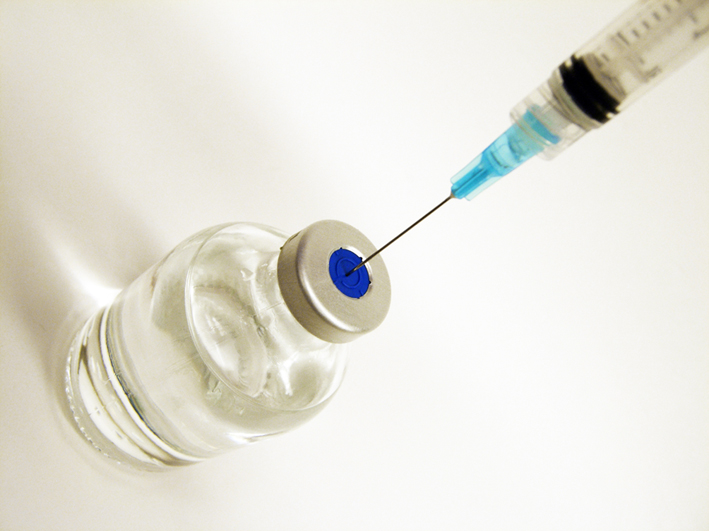
Infectious diseases kill millions of people every year and children under the age of five, especially those in developing countries, are particularly vulnerable. While significant progress has been made through global immunization campaigns to protect against many of these diseases, the delivery of vaccines to target populations is costly and requires considerable logistical support. All vaccines currently recommended by the World Health Organization (WHO) to treat these diseases – DTP (diphtheria, tetanus, pertussis (whooping cough)), BCG (tuberculosis), oral polio vaccine (poliomyelitis), yellow fever (for countries at risk) and measles – require cold storage (between 2ºC and 8ºC) to remain stable and viable. This can significantly increase the cost per dose of these vaccines and can result in significant vaccine wastage arising from breaks in the cold chain. Equally, if not more importantly, it leads to a significant proportion of ineffective vaccines being administered with consequent loss of life. Some estimate that up to 40% or more of vaccines are ineffective in sub Saharan Africa.
Last year, British scientists made a breakthrough that “offers a simple and cheap way of making vaccines stable – even at tropical temperatures”, using a technology that promises to “revolutionize vaccination efforts”.
In early 2010, scientists from the Jenner Institute, University of Oxford and from the British company Nova Bio- Pharma Technologies, carried out a proof-of-concept study showing that vaccines they are developing can be stabilized for months using Nova’s patented Hypodermic Rehydration Injection System (HydRIS).
“Currently vaccines need to be stored in a fridge or freezer. That means that you need a clinic with a nurse, a fridge and an electricity supply, and refrigeration lorries for distribution,” explains Dr. Matt Cottingham of the Jenner Institute at the University of Oxford, lead author of the study. “If you could ship vaccines at normal temperatures, you would greatly reduce cost and hugely improve access to vaccines,” he says. “You could even picture someone with a backpack taking vaccine doses on a bike into remote villages.”
The team demonstrated that two different virus-based vaccines could be stored on sugar-stabilized membranes for 6 months at 45ºC without any deterioration. When stored at 37ºC for 12 months, these vaccines showed only very small losses in the amount of viral vaccine re-obtained from the membrane.
“We’ve shown that a very simple way of heat-stabilizing vaccines works for two viruses that are being used as the basis for novel vaccines in development,” Professor Adrian Hill of Oxford University observed. “This is very exciting scientifically, because these viruses are fragile. If we are able to stabilize these, other vaccines are likely to be easier.”
The vaccines use live viruses that have been engineered so that when they enter the body they stimulate an effective immune response without replicating and causing infection. A long shelf life for live-virus-based vaccines is therefore desirable when delivering these vaccines to patients in remote areas.
Using this method, vaccines are mixed with the sugars trehalose and sucrose. This mixture is left to dry slowly on a filter or membrane where it eventually solidifies to form a thin sugary film.
This preserves the active part of the vaccine “in a kind of suspended animation” and protects it against deterioration even at high temperatures. By flushing the membrane with water, the vaccine is instantly rehydrated. “The beauty of this approach is that a simple plastic cartridge, containing the membrane with vaccine dried on, can be placed on the end of a syringe,” explains Dr. Cottingham. “Pushing a water solution from the syringe over the membrane would then release the vaccine and inject it into the patient.”
For Nova’s Managing Director, Peter White, “this new technique of drug delivery is one of the most exciting developments in the British pharmaceutical and biotechnology industries, especially as it can be used for highly unstable products, for instance vaccines for malaria.”
Preparing vaccines that do not require refrigeration has been identified as one of the major unsolved problems of global health. Maintaining the cold chain for vaccines costs millions of dollars a year. In 2000, the total annual expenditure on immunization for low-income countries averaged US$6 per live birth. By 2010, the cost of immunization with traditional vaccines as well as hepatitis B and Hib (haemophilus influenza type b) vaccines reached US$18 per live birth according to the WHO.